Healthgen Biotechnology, specializing in molecular pharmaceutical research and development, is focusing on developing and manufacturing a series of animal-free and safety products by using the state-of-the Art platform called OryzHiExp, having high capacity to express proteins or small peptides in the rice grain. Using the OryzHiExp platform, Healthgen Biotechcan provide customized protein or peptides expression and purification services. The process route and infrastructure of large-scaled protein purification has been established, and can implement the industrial expression and production requirements.
Healthgen Biotech has strong technical force, advanced and complete protein expression and purification technology research and large-scale production facilities, can provide high capacity and significant advantages alternative development and manufacturing approaches.
Rice endosperm platform provides a new approach to produce animal component free, environment fridently, high bioactivity and biological safety recombinant proteins or peptides. Also, Healthgen Biotech is offering a system that can be easily scaled up to have high capacity production.
Healthgen Biotech can provide full contract development and manufacturing services from the early stages of protein expression, product selection, purification and optimization to full-scale product launch, all in Healthgen Biotech facility.
Healthgen Biotech’s technology platform and facility capabilities are applicable to development of vaccines, antibodies, therapeutic proteins, industrial biological enzymes, medical devices.

The pilot factory have a comprehensive factory management system and well-established production equipments, includes extraction unit, purification unit, filtration and ultrafiltration Unit and lyophilization unit. The extraction unit is composed of 3 sets of 1000L extracting tanks, pipeline system, sanitary centrifugal pump, frame filter press and cartridge filter. The chromatographic purification system is GE advanced AKT system, and also can match with other chromatography columns diameter from 100-800mm, such as BPG series, VERSA 450 and so on. The production capacity can reach 4-5kg per batch.

The one all set ripe and complete equipments enable one batch capacity can reach 10+ kg recombinant proteins or peptides per batch. The chromatographic purification equipments are 5 sets of chromatography system. Each chromatographic system is consist of control system (AKTA Process 1.0 inch) and a chromatography column (AxiChrom1000mm). Sterile filling line can be applied to 50mL and 100mL bottle filling; and the production capacity reaches 3000~6000 bottles per hour.
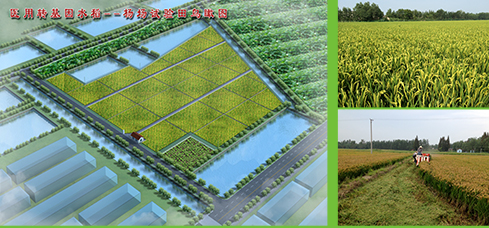
The transgenic rice production farm is managed under GAP guidline. One season yield of brown rice can reach 200,000kg. There is an isolation zone that is more than 100m apart from non-transgenic rice, a buffer zone around the field (1.5 m), fencing with a vedio monitoring in the field trail at whole rice growth stages and has established standard operation protocols for sowing, planting, harvesting, drying, transporting, processing and storage etc.

Provide quality study, analytical method development and analytical method validation, including DNA quantitative analysis, protein content, metal element analysis, ELISA detection, osmotic pressure, particle analysis, isoelectric focusing, molecular weight determination, peptide mapping, microbial limit test, sterility test.



