A powerful alternative to solve the issue of BSA impurity residue -plant-derived recombinant human serum albumin “Hywill™ OsrHSA”
In the In Vitro Diagnosis ( IVD ) industry, BSA(Bovine serum albumin) has been one of the widely used raw materials. It plays an important role in various diagnostic methods, such as biochemical, chemiluminescence, mass spectrometry and other detection methods. However, BSA is mainly extracted from bovine plasma. The residual IgG, protease, hormone, fatty acid and other substances in serum will still have trace residues even after the relevant purification process. In some blood samples, especially trace samples, it may interfere with the results and become a major issue in IVD detection.
Recombinant human serum albumin (Hywill™ OsrHSA ) specially developed for IVD detection, is a recombinant human serum albumin OsrHSA derived from rice endosperm cells. Due to the production method of plant sources, and after strict extraction and purification, it does not contain the above-mentioned interfering substances. According to internal self-test and customer feedback, Hywill™ OsrHSA can be used for BSA-related applications in the IVD industry, such as protective agent, blocking agent, buffer solution, plasma matrix standard preparation, etc.
What is Hywill™OsrHSA?
Hywill™OsrHSA, is a plant-derived recombinant human serum albumin independently developed and produced by Wuhan Healthgen Biotechnology Corp.. Compared with BSA, it is expressed by bioengineering technology, not derived from animal serum extraction, with high purity, excellent batch consistency. With the increasing demand for HSA Today. Hywill™OsrHSA is undoubtedly the surging power of the IVD industry!
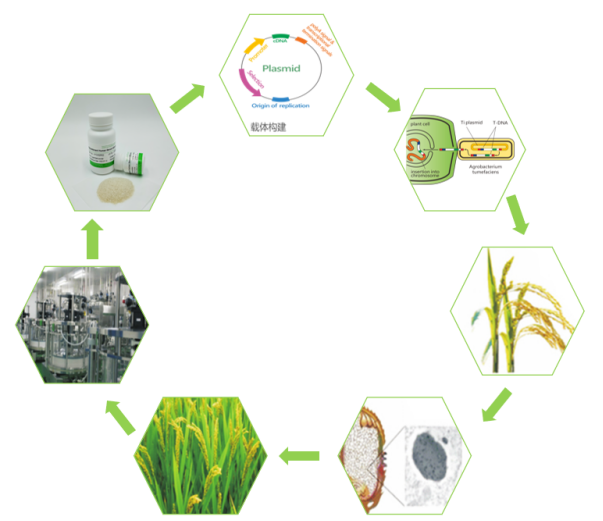
In addition, unlike traditional human serum albumin extraction, Hywill™OsrHSA is collected and purified from genetically engineered rice endosperm cells after expression. The whole process fundamentally solved the residue of blood source components and improved the overall purity of the product. At the same time, the large-scale planting-harvesting-purification production process (As shown below) makes Hywill™OsrHSA have highly consistent batch-to-batch repeatability, overcomes the drawbacks of multiple tests in different batches, and also avoids the instability of microbial recombinant protein fermentation broth and many impurities. It can better match large-scale production use.