Recombinant Human Serum Albumin(OsrHSA) Derived From Oryza Sativa Has Been Approved By CFDA For The Clinical Trial In China
OsrHSA, a recombinant human serum albumin derived from transgenic rice seeds has gained the approval of the clinical trial from Chinese Food and Drug Administration (CFDA) at 28 April, 2017. It is a milestone event in the research and applications of plant bioreactor, which provides a new approach for protein drug production in the future.

Therapeutic HSA is primarily derived from donated human blood, which is mainly used in the treatment of liver cirrhosis, hemorrhage shock, severe burn, scald, plastic surgery, hypoproteinemia, renal edema etc. Currently, the demand of HSA is about 850 tons per year worldwide, and 420 tons per year in China. In recent years, due to shortage of blood plasma, HSA supply is shortage, particular in China and Asian countries, 60% HSA demand depend on the imports in China. Simultaneously, the threat of blood borne diseases such as AIDS and hepatitis has become the more and the more concerns. So, FDA and EMEA explicitly encourage the application of the recombinant HSA with animal component free.
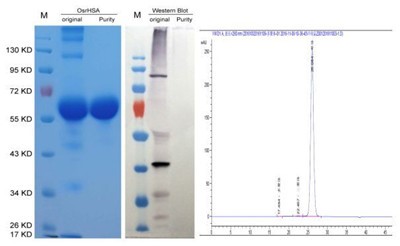
Given its high demand, Scientists had been attempting to use recombinant technology for decades to produce recombinant HSA in various organisms such as bacteria, yeast, tobacco and even cows. However, it had been difficult to produce cost-effective and non-immunogenic HSA suitable for large dosages used in treatments. Thus far, there had been no attempts on human trials with recombinant HSA. Dr. Daichang Yang, professor of Wuhan University and president of Healthgen Biotechnology Corp. with his colleagues established a rice endosperm specific expression technical platform-OryzHiExp to highly express recombinant HSA. After more than decade efforts, a recombinant human serum albumin derived from rice (OsrHSA) has been approved by CFDA for clinical trial in China.

It is a milestone event in plant molecular pharming. If the trials using rice-seed bioreactor are successful, it will provide an alternative approach to largely produce pharmaceuticals with cost-effective approach. Furthermore, as soon as OsrHSA will be verified to be safe in human beings, it will open light for plant molecular pharming and alleviate the apply shortage of HSA in China, even in the world.





